Newest
This cancer and malignant tumor early screening and diagnosis company has received CE certification for seven products in 2 years!
 2022-05-27
2022-05-27
Cancer is a major global public health problem. In 2020 ,10 million people worldwide has been dead of cancer ,which is the second leading cause of death globally, It accounts for one sixth of the total deaths. However, for almost all cancers, if detected, diagnosed and treated early, patients' chances of survival are bound to increase significantly. As a global leader in early cancer screening and diagnosis, Wuhan Ammunition Life-tech Co., Ltd. has always been committed to the global promotion of early cancer diagnosis technology and products. It adheres to the product concept of being early, accurate, convenient and inclusive. It has created and constructed a new generation of cancer screening and testing technology platform and product system. It has launched a series of non-invasive early detection products covering digestive system tumors, gynecological tumors, tumors of the urinary system and other tumors.
It is reported that in the past two years, the seven early non-invasive detection products for digestive system tumors and gynecological tumors developed by Life Technology have passed the EU CE certification, and can be distributed and sold in EU countries and countries that recognize the EU CE certification for advanced cancer early screening technology. This marks a major step forward in the globalization of Life Technology's cancer early detection technology and products, and also validates Life Technology's hardcore strength in R&D innovation and product registration.
The seven CE-certified fluorescent PCR DNA tests include: Stool DNA Methylation Detection Kit for Colorectal Cancer, Plasma DNA Methylation Detection Kit for Colorectal Cancer, Plasma DNA Methylation Detection Kit for Esophageal Cancer, Plasma DNA Methylation Detection Kit for Liver Cancer, DNA Methylation Detection Kit for Cervical Cancer, DNA Methylation Detection Kit for Endometrial Cancer and Plasma DNA Methylation Detection Kit for Digestive Cancers.
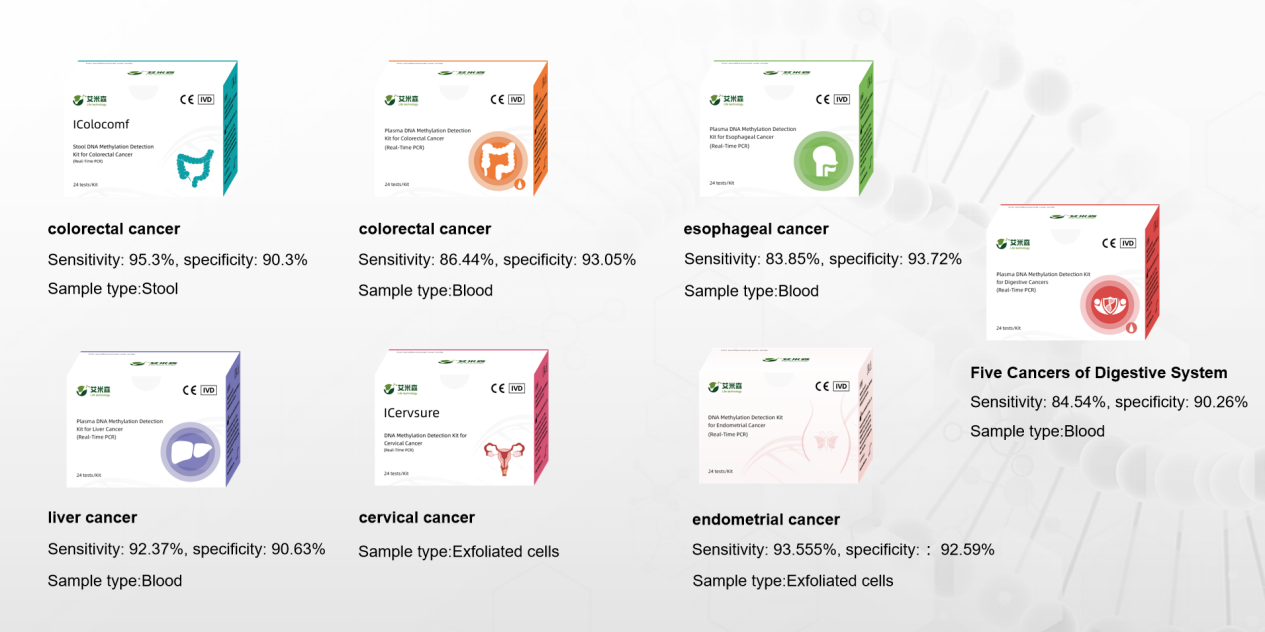
Test performance of 7 CE certified kits
Company:Wuhan Ammunition Life-tech Co., Ltd
Contact Person: Ryan Chen
Email: chenbj@whammunition.com
Website: www.ammulifetech.com
Telephone: +86 18674010656/+86 13168856463
Location:No. 818, Gaoxin Avenue, Wuhan East Lake New Technology Development Zone




